Patients like Niva fight to improve their quality of life every day with help from the medical and pharmaceutical industries.
This network diagram highlights Niva's journey and expands to reveal how the Charles River Microbial Solutions portfolio is contributing in every area within the quality control testing and manufacturing processes to bring safe, effective therapies to patients.

The ability to detect all environmental Gram-negative bacterial endotoxins is an important element of safe pharmaceutical production, medical device manufacturing, radiological health, and dialysis water testing fields. Highly sensitive and exquisitely specific in vitro assays are necessary to ensure products are safe for release, and alternative, non-compendial assays can return false negatives –potentially subjecting patients to a fatal pyrogenic reaction. With over 70 million tests performed annually, the LAL assay delivers confidence with every test performed. As a cGMP, FDA approved and licensed therapy manufacturer, the organization you partner with for your solutions should be held to those same standards too.

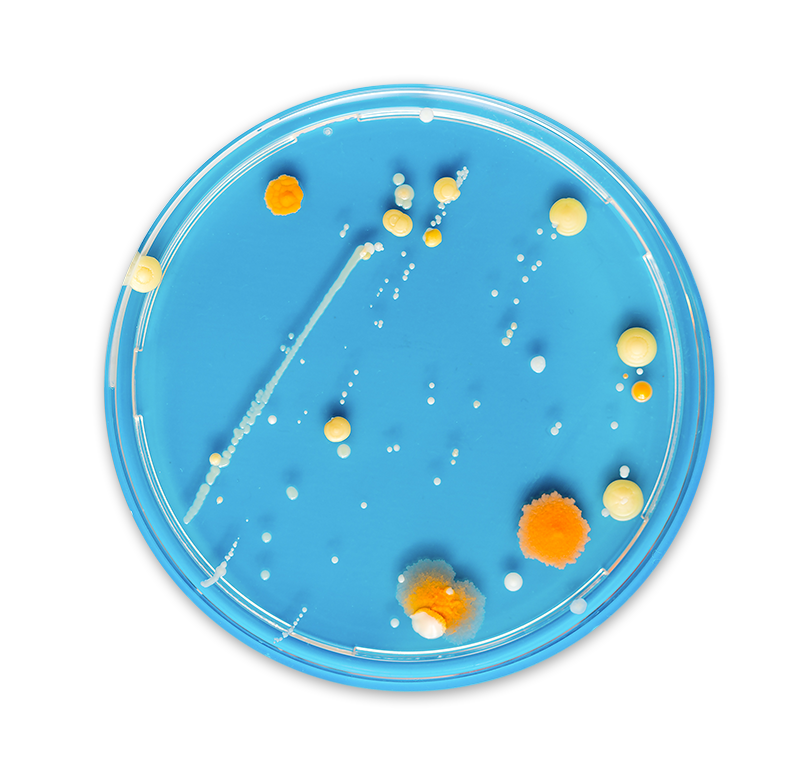

The safety of your manufacturing environment depends on a clear picture of your microbial identification data. Without confidence in your environmental monitoring (EM) data, you can never be completely sure that your products are free from unwanted microorganisms. Consistent and diligent EM practices are some of the best strategies to achieve operational improvements that eliminate risk to patient health. Accurate and timely microbial identification is crucial for tracking and trending EM data that guide critical decisions and protect you from releasing contaminated products.

Assays that require a visual read for final sterility confirmation are no longer acceptable. Traditional methods don’t help you release product faster, detect contamination sooner, or eliminate all doubt when answering the question “is it safe?” Rapid microbiological methods (RMMs) are effective at modernizing the laboratory by transforming subjective decisions into unambiguous results. In an industry that relies heavily on cutting-edge technology, should final sterility really be proven with a visual turbidity check? With over 15 million sterility tests performed every year, it only takes one bad call to jeopardize countless lives.
